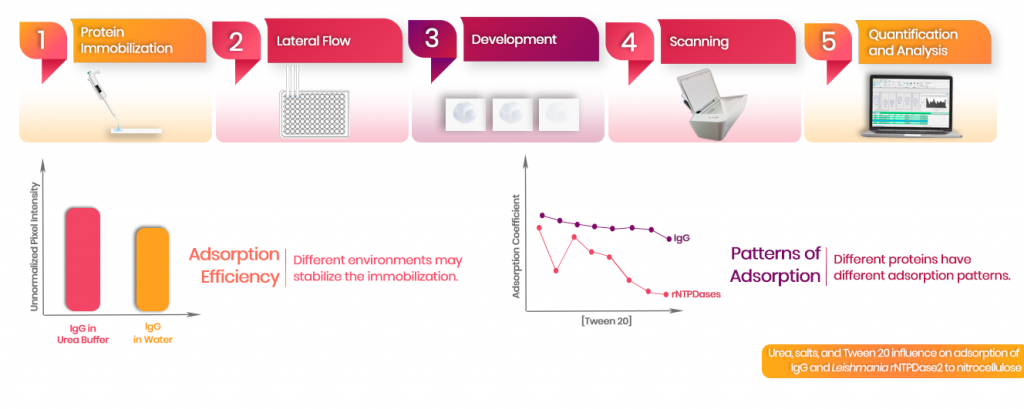
Urea, salts, and Tween 20 influence on adsorption of IgG and Leishmania rNTPDase2 to nitrocellulose
Raissa Barbosa de Castro Anna Cláudia Alves de Souza Nancy da Rocha Torres Pavione João Victor Badaró de Moraes Isadora Cunha Ribeiro Joice de Melo Agripino Gustavo Costa Bressan Raphael de Souza Vasconcellos Abelardo Silva-Júnior Juliana Lopes Rangel Fietto
Abstract
Lateral flow immunochromatography is a widely used technique for immunological assays. Construction of test and control lines is mostly done by antigen adsorption to nitrocellulose membranes, a process not fully understood. This study aimed to evaluate the influence of urea, salts, and Tween 20, on adsorption. The performance of canine IgG in water and in buffer containing urea and salts (pH 8.3) were compared to observe if the interferents would lead to protein stripping when challenged with increasing concentrations of Tween 20 in the lateral flow buffer. Immobilization of the rLiNTPDase2, an antigen for Canine Leishmaniasis diagnosis, was evaluated and compared to the rLbNTPDase2 by the same method. There were no differences between adsorption coefficients of IgG in water and in buffer, but high salt and urea concentrations seems to stabilize and enhance IgG immobilization. Adsorption performance between canine IgG and rNTPDases had different patterns, but was highly similar between rNTPDases, indicating that protein identity may have an important role. Also, low concentrations of Tween 20 in the flow solution may aid the maintenance of rNTPDase2 on the strips. Our results bring insights about protein adsorption and perspectives about the influence of urea, salts and Tween 20 on this process.